TNT (trinittotoluena): Understanding, Images, Adnature, Utility and Danger
TNT understanding
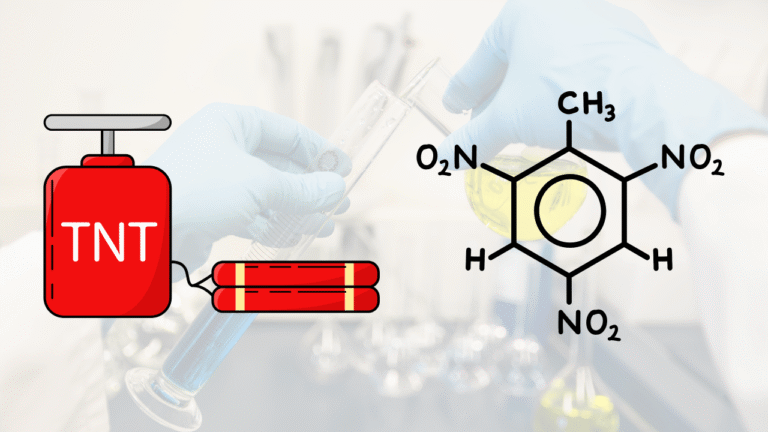
TNT, or Trinitrotoluena, is an organic chemical compound with molecular formula ♪. This compound is the derivative of the Toluena that contains three nitro clusters, and is known widely as high-powered explosives. In nomenclature IUPAC, TNT is referred to as 2,4.6-trinitrotuluena.
TNT was first synthesized by Julius Wilbrand in 1863 in Germany. Initially, this compound was used as a yellow dye, until the end of the 19th century was discovered that it has a strong, stable, and can be melted easily without exploding. This is what makes him popular in military and industrial applications.
Chemical Structure and Image Molecules
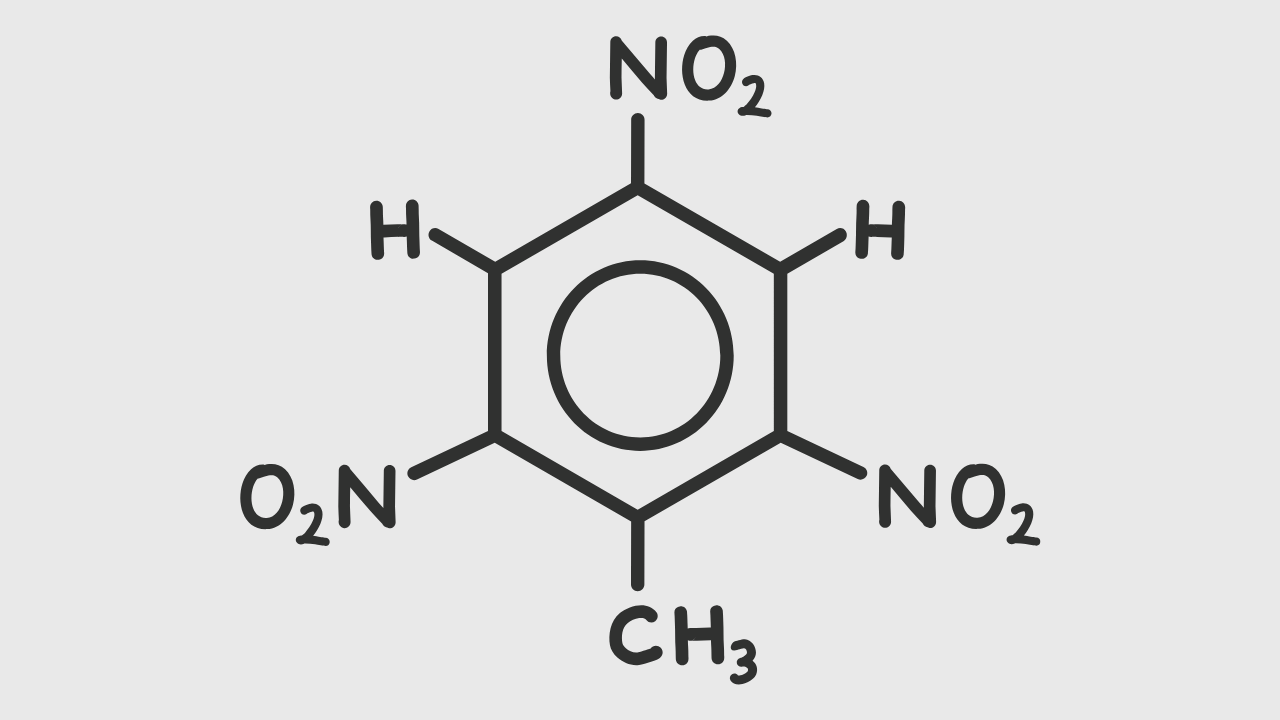
TNT chemical structure composed:
- One ring benzene (aromatic)
- Three nitro groups in orto and para (2, 4, and 6)
- One methyl cluster.
This structure creates TNT:
- Characteristic aromatic stable
- Rich oxygen from nitro groups
- It produces high energy when it experiences explosive decomposition
Draw molecular structure:
Physical and TNT Chemistry
Physical nature:
- Shine yellow crystal solids
- Flow: 0,80.1 ° C
- Flash point: 0,295 ° C (non-flammable)
- Densitas[1.65 g / cm]
- Not immersed in water, but dissolved in organic solvent like acetone, benzene, and etanol
Chemical nature:
- Stability: Pretty steady against mild mechanical shocks and friction
- Sensitivity: Need a detonator to explode (not explode only with heat)
- Reaktivitas: Can experience reduction into a toxic aromatic amin compound
Why would TNT explode?
TNT explosion is the result of Exothermic decomposition From an oxygen-rich nitro group. When detonated, TNT suffered rapid reactions that produce gas like nitrogen, carbon monoxide, and water vapor (H), all in large quantities and high pressure.
The reaction can be simplified as follows:
2 C S01E0
Gas generated in large volumes causes a destructive shock wave, that's what's called an explosion. TNT is selected in many applications because it can be stored and transported safely, and it only explodes when it is stimulated.
TNT Utility
1. Military
- Used as the main ingredient in artillery, mines, bombs and grenades
- Mixed with other ingredients such as RDX or aluminum to produce explosive composite Like amatol and pentolite.
2. Industry and Mining
- Used in rock-blasting activities in mines and big construction projects
- Although its use is decreased due to environmental regulation, TNT is used due to its ability to form in explosive printing
3. Default Exploding Size
- TNT used as Standard energy measurement explosion, for example: 1 ton of TNT
- TNT megaton units are used in measuring nuclear explosion and volcanic eruptions.
Danger and risk health
1. Toxicity
TNT known Poisoned to humans And animals. Chronic or acute exposure can cause:
- Skin irritation and skin color changes to brass (called "yellow joundice")
- Heart and spleen damage
- Hemolithic anemia
- Reproductive system function disorder
In World War I, a lot of female workers at the munitions plant experienced skin changes from constant exposure to TNT, so called "canary girls."
2. Karsinogenicity
Some toxicology studies mentioned that long-term exposure to TNT is potentially carcinogenic karena disebabkan oleh jenis bahan kimia Carcinogen, although it's still in the study phase. In lab animals, observed tumor growth after repeated exposure.
Environment Impacts
1. Contamination of Land and Water
TNT can pollute the environment through:
- Explosive factory waste
- The rest of the explosion in the military field.
- Old ammunition decay
It's hard to unravel naturally and it can accumulate on the ground, sediment, and soil water. The exposure to the water environment even caused death on acidic biota.
2. Remediation Process
TNT-contaminated soil or water reladiation involves:
- Bioremediation with nitroaromatic degrading microorganisms
- Photo with UV light
- Adsortive filtration using active carbon
However, this method is still expensive and challenging, especially in a large contamination area.
Conclusion
TNT is an important nitroroaromatic compound that contributes to major military and industrial contributions. His stable, processed, powerful characteristics make him very popular as explosives.
However, under his superior authority, TNT keeps a serious potential for human health and environmental harm. Therefore, its use now began to be restricted and replaced by more eco-friendly explosives.
The profound understanding of structure, nature, and impact is very important especially for chemical professionals, the environment, and the military are directly connected to this compound.
FAQ (Frecently Asked Questions) about TNT
TNT used to what?
TNT is used as high-powered explosives in various applications, especially in military and industrial fields. It's common in artillery bullets, bombs, landmines, and mines because it's stable, easily handled, and powerful.
What's the trinitrotuluena use for?
Trinitrotuluena is used as the main component in ammunition and explosives because it has a low melting point, but it's not easy to detonate without detonators. It allows melting and casting into the casing without risk of spontaneous detonation.
What's the use of a trinittotoluena compound?
In addition to military use and industrial detonation, trinitrotuluena also acts as a standard ingredient in measuring the force of the explosion. Energy units like "ton of TNT" or "megaton TNT" are used on a large scale, for example, to measure the power of nuclear bombs or volcanic eruptions.
Why would TNT simply explode?
TNT does not include very sensitive materials, so it doesn't easily explode just because of heat or friction. However, when given a trigger like the detonator, it experiences a rapid chemical reaction that produces massive amounts of gas and high pressure, causing a powerful explosion.
What's TNT stand for?
TNT stands for Trinitrotuluena, an aromatic compound made of benzene rings that are tied to three nitro clusters and one methyl clusters.
What's TNT stand for?
The TNT complex in the chemistry context is 2,4,6-Trinitrotuluena, which is systematically referring to the nitro group position on the benzene ring that the molecule has.
Source:
- Encyclopaedia Britannica
Full explanation of the nature, history, and use of TNT in military and industrial fields. - Public
The chemical database of the National Institute of Health that contains structure, toxicity and physical properties of TNT.