Benzoat Acid Consciousness, Other Names, Adnature and Example

Benzoat acid It's one of the simple aromatic compounds that matter in organic chemistry, whether it's from the side of molecular structure, reactivity, or its applications in various industries. It talks deeply about the understanding of benzoic acid, synonym or any other name used in scientific literature and industry, the physical properties of its own possession, and some examples of its use, based on literature studies and experimental data from various trusted scientific sources.
Benzoat Acid Understanding
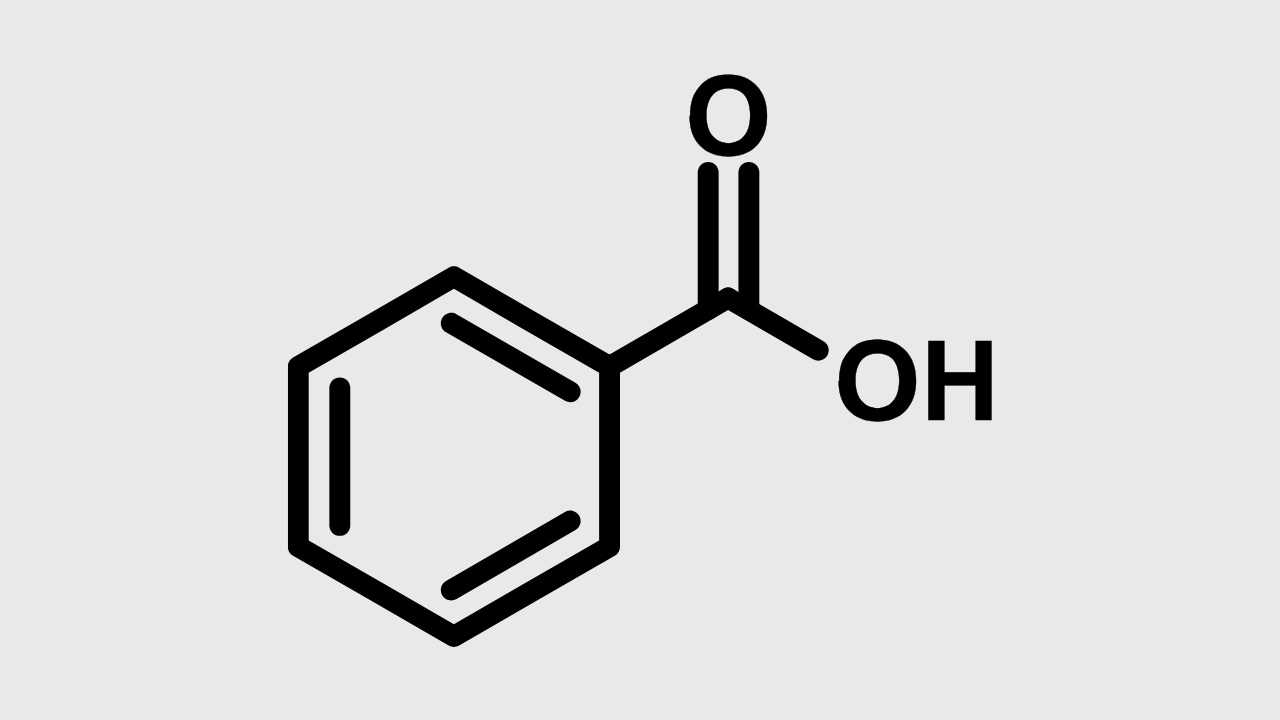
Benzoate acid is an organic compound with chemical formula. C-H-COOH. Structurally, this compound consists of a benzene ring bound to a carboxic (-COOH) cluster, making it as acid aromatic monocarboksilate. Benzoate acid is a member of a simple carbochilate acid class and many are found naturally in plants and animals.
Historically, benzoat acid was first isolated in the 16th century from benzine gum, which is the aromatic sap of a tree. Styrax Shh. The name "benzoat" comes from that natural material. As the growth of modern organic synthesis, the acid of benzoate is now produced industrial through the oxidation of toluena using the metal catalyst.
_
In various fields and literature, benzoat acid is known as several other names, among other things:
- Fenilformat acid
- Carbochibenzene acid.
- E210 (as food additive code in international notation system)
- Benzencarboksilat (in IUPAC nomenclature)
This synonym is often used depending on the context of its use, as in pharmaceutical, food industry, or theoretical chemistry.
Physical and acidic properties of Benzoat Acid
1. Physical properties
- The molecular formula: ♪
- Molar mass: 122.12 g / mol
- Bow: Solid white chrystalin
- Melted Point: 122 ° C
- Boiling point: 249 ° C (with partial decomposition)
- It's in the water: 3.4 g / L at 25 ° C (low)
- The high-quality awkwardness of ethanol, ether, and other organic solvent.
2. Chemical nature
- Benzoate acid is a weak acid with pKa around 4.2, so that only partly ionized in water solution.
- In the base state, the benzoat acids form salt benzoat, like sodium benzoat.
- It can have an aromatic substitution reaction (for example, nitrate, sulfonation) in an orto-para position.
- Can experience decarbochilation at high temperatures into benzene and karbon dioksida, a reaction relevant in another aromatic compound synthesis.
Benzoat Acid Production (Industrial Scale)
Commercially, benzoat acid is produced in a way toluena catalytic oxidation in liquid phases using air or molecular oxygen as oxidizing and coalted-based catalyst or mangan. This reaction can summarize as follows:
Come on.
This reaction has a high rendering and is widely used in industry chemical material basic due to efficiency of process and low production costs.
Benzoat Acid Usage Example
1. Food Industries
The acid benzoate and its derivative (mainly sodium benzoat) used as Food preservatives Code E210 to E213. This compound is active against microorganisms like yeast and bacteria in low pH conditions. Used in products like:
- Soft drink
- Fruit juice
- Sauce and vinegar
- Margarin
2. Pharmaceutical Industries
Benzoate acid is used as active material or exipien in:
- Salts and antifungal cream
- Exspectoran medicine.
- Topic antiseptic
3. Organic Senyawa Synthesis
The benzoate acid is an important precursor in the thesis of the benzoate, benzoat benzoat, and other intermediate compounds used in the perfume industry, plasticizer (e.g. plasticizer), and synthetic dye.
Four. Plastic Industries and Resin
The derivative of benzoic acid, such as benzoil chloride and anhidrida benzoat, is used in production. resin alkid, polyester, and plasticizer to increase product flexibility.
Risk and Security
Based on the evaluation of Food and Drug Administration (FDA) And other regulatory bodies, benzoate acid is safe when used within designated limits. However, excessive consumption or use in certain conditions can result in side effects, such as:
- Skin irritation and respiratory tract if inhaled as fine powder
- Formation benzene (carcinogen) if it reacts to ascorbat acid (vitamin C) under certain conditions
Therefore, strict regulation applied to its use, including maximum residue limits in food products (generally < 0.1%).
Supporter Experimental Data
In publication by J. Agric., said that benzoic acid is naturally detected in fruits such as cranberries, plums, and apples with varied concentration of 0.05-0.5 mg / g. The study uses a high-performance liquid chromatography (HPLC) to authenticate, indicating that although natural, benzoat acid must still be controlled by its exact use in food formulation.
Conclusion
Benzoate acid is an important aromatic organic compound that has a crucial role in the food industry, pharmaceutical and chemistry. With the nature of the weak acid, the thermal stability is good, and the ability to form salts that dissolve water, benzoic acid becomes the primary option as preservative and chemical precursor. The profound understanding of the chemistry and its applications is important not only to academics, but also to the industrial offenders who implement them on a massive scale.
As researchers and chemical practitioners, we are charged not only to understand the molecular structure and its reactiveness, but also to note the toxicology and environmental impact of this compound, especially if used in large and long-term quantities.
Scientific Reference:
- WHO / JECFA: Benzoat Acid Profile
- WHO / JECFA: Benzoat Cover (Benzoic Acid & Salts)
- Scientific Journal (J. Agric.
- Scientific opinion of European Food Safety Authority (EFSA)
- European Commission: von Opinie Security Adapif Documents · Global Voices
General Question About Benzoat Acid
What's benzoic acid?
The benzoate acid is an aromatic organic compound with a chemical formula C: This compound is naturally found in some fruits and is used widely as a preservative of food and materials in the chemical industry.
What do you mean by benzoat acid?
It's a white crystal chemical compound that has a weak acid properties. Benzoate acid is used to prevent growth from microorganisms in food and drink as well as the material between making other chemicals.
Is that benzoat and its function?
Benzoat is salt or ester of benzoat acid, like sodium benzoat. It's mainly as an effective preservative to prevent growth of bacteria, yeast and fungi in sour food products and drinks.
What's the use of benzoic acid?
Benzoate acid has a variety of uses in industry. This compound is used as a preservative of food and drink because it is able to inhibit the growth of microorganisms. In addition, benzoic acid is also used in topical drug formulation, cosmetic products, and as a material between the synthesis of other chemical compounds like trists and resins.
What's the advantage of benzoic acid?
The main advantage of using benzoic acid is its effectiveness in extending product savings, especially food and drinks. Benzoate acid is stable, it has low toxicity if used in a safe limit, as well as easily formulated with other materials. This makes it one of the most globally used preservatives.
What preservative benzoate?
Benzoate acid is used as a preservative in various foods of food and drink like soft drinks, fruit juice, tomato sauce, pickles, margarine, and jam. It's normally used in salt, like sodium benzoate, because it's easier to dissolve in water and more effective in low pH conditions.