Sublimation, Sample, Destination, Processing

Do you know what that means? sublimation? So sublimation is a term in chemistry that deals with the shape change from a substance from solid to gas or from gas to solid without through liquid form. Sublimation is also one method of separation Chemical alloy based on the differences of the seething matter.
Sublimation Sample
One of the easiest examples of sublimation is in the process of making mothballs. The mixture of mothballs and charcoal is heated so that petrified chalk will evaporate. After that, chalk vapor cooled so it became solid again.
Another example of sublimation is:
- The dry ice evaporates into carbon dioxide gas without melting first.
- Iodium (iodine) which goes from solid to purple vapor without melting first.
- Naphtalen that goes from solid to steam without melting first.
Sublimation Destination
The purpose of sublimation is to acquire a pure substance or some pure substance from a mixture called purification. For example, to separate the iodium from its toner mix to get pure iodium.
Another purpose of sublimation is to know the existence of a substance in a sample by way of a laboratory analysis. For example, to identify organic substances that can be sterilized.
Sublimation Process
The sublimation process can generally be done in two ways, which is natural sublimation and artificial sublimation.
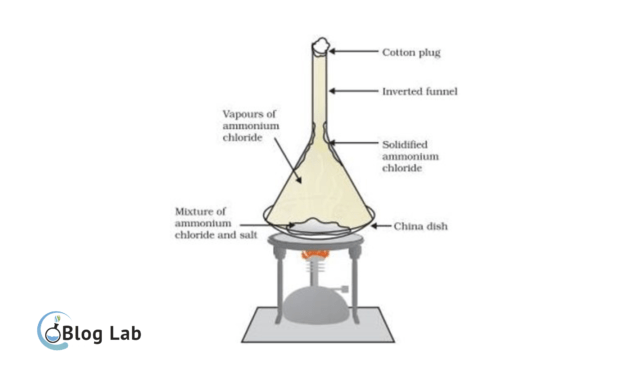
Natural sublimation is a sublimation process that happens naturally as a result of the natural process itself. For example, the sulphur sublimation that happens on the bottom-crater volcanoes.
Artificial sublimation is a sublimation process that happens intentionally or forcibly, both on an industrial scale and in a laboratory. The principle of artificial sublimation is to heat up solid substances that allow it to become gas or steam. Then, the gas or the steam is packed and cooled or condensed back to denser.
Artificial sublimation procedures on a laboratory scale are as follows:
- Prepare tools and materials as needed, like a steaming dish, a glass cup, a spatula, a watch glass, a funnel or a pumpkin containing water as a coolant, and a hot source.
- Insert solid matter that will be sublimated into a steamer dish. Cover the steamer dish with a watch glass. Put a funnel or pumpkin with water on top of the watch glass as a cooler.
- Heat a steaming chalice with a small slow fire. The denser will vaporize, while the mixer remains dense.
- The steam formed because of the cooling process will turn back into a dense one attached to the cooling wall.
- If there's no more irreversible substances, stop the warming process and let the cold get the vapors in order for the vaporization to be formed. Then, collect the substance formed on the cooling device for her purity. If less pure, the sublimation process can be repeated until it gets a pure substance.
Conclusion
Sublimation is the changing of matter from solid to gas or from gas to solid without through liquid form. Sublimation is also a chemical mixed separation method based on the different boiling points between mixed substances. The purpose of sublimation is to get a pure substance or to know the existence of a substance in a sample. The sublimation process can occur naturally or artificially. Artificial sublimation process can be done by heating up solid substances that allow it to become gas or steam, and then holding and cooling or condensing the gas or the steam becomes dense.
That's a brief review of the understanding of sublimation, example, purpose and process. Hopefully this article will provide useful and exciting information for you. Thank you for reading this article out. See you in the next article.
Articles quoted from sources:1. doseneducation .co.id 2.