What is the Single Kovalen Binding?

When learning about chemistry, one of the important concepts that needs to be understood is Chemical bonds. Chemical bonds help us to understand how atoms are tied to each other and form different molecules and compounds. One of the most common chemical bonds is a single covalent bond. In this article, we're going to talk deeply about what a single covalent bond is and how this bond is involved in forming the compounds that we know it.
What is a single covalent bond?
Ikatan kovalen single happens when two atoms share an electron pair to form a stable molecule. It's different from an ionic bond, where electrons are transferred from one atom to another. In a single covalent bond, both atoms contribute to a shared couple of electrons so they both gain a more stable electron configuration.
How does a single covalent bond form?
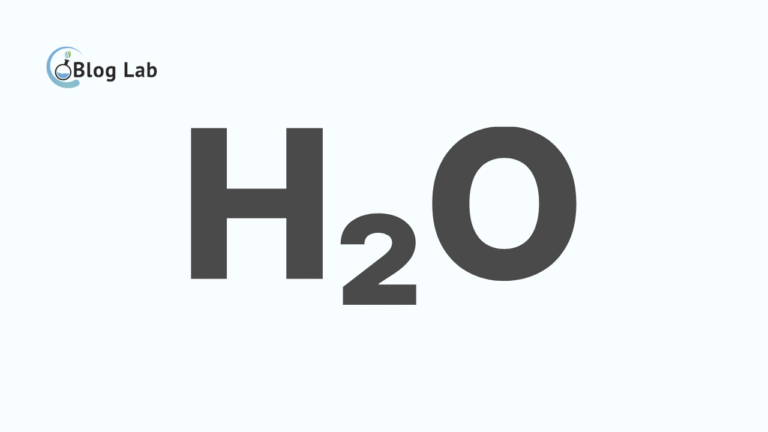
A single covalent bond formed when two nonmetal atoms shared electrons in an attempt to reach a more stable electron configuration. In this process, both atoms draw electrons at themselves, creating an electric field called covalent fields. Electrons will be distributed in covalent fields, creating strong bonds between the two atoms. It creates a stable molecule, such as in a molecule of H2O that is formed from a bond between oxygen and two hydrogen atoms.
Single Covalent Binding Sample
Single covalent bonds occur in various organic and inorganic compounds. The most common example of a single covalent bond is on a glorious gas compound, like neon, argon and Kryptonian, where all the valence electrons pair up and are in stable condition.
Single Kovalen Binding characteristic
A single covalent bond has some characteristics that make it unique. First, a single covalent bond only occurs between nonmetal atoms, because metal atoms tend to release electrons to form positive ions. Secondly, single covalents bond is very strong and hard to break, because electrons are divided by one another. Third, molecules that form a single covalent bond tend to have a stable structure and are not easily unraveling.
Single Kovalen Binding Utility
Single covalents are essential in forming organic and inorganic compounds that are used in everyday life. For example, single covalents are used in making fossil fuels such as gasoline, oil and natural gas.
The role of Single Covalent Binding in Life
Single covalent bonds play a crucial role in our lives. They form organic and inorganic compounds that form the basis for things, from the food and drinks we consume to the fuels we use to move the vehicles. Single covalent bonds also form compounds in the human body, like proteins and DNA.
Conclusion
In this article, we've discussed what a single covalent bond is, how these bonds form, a single covalent bond example, characteristics and their use. Single covalents are essential for forming organic and inorganic compounds that are used in everyday life, and have an important role in understanding chemistry. By understanding a single covalent bond, we can understand how atoms-atoms bind to each other and form molecules and compounds that form the world around us.
FAQ
- What's the difference between a single covalent bond with a double covalent bond?
- Single covalent bonds occur when two atoms share one couple of electrons to form a stable molecule, while double covalent bonds occur when two atoms share two pairs of electrons.
- What's a covalent polar and nonpolar bond?
- Covacent polar bonds occur when two atoms share electrons in an unbalanced way, so one atom pulls electrons more strongly than the other. nonpolar covalent bonds occur when two atoms share electrons equally.
- What example of organic compounds formed from a single covalent bond?
- An organic compound example formed from a single covalent bond is methane (CH4) and ethana (C2H6).
- Why is a single covalent bond so hard to break?
- Single covalents are hard to solve because electrons are divided by pulling each other, so covalents are forming very strongly.
- How does a single covalent bond play in forming compounds in the human body?
- Single covalent bonds form compounds in the human body, like proteins and DNA, which are essential components in the formation and maintenance of cells and tissue.